By Tom Latek
Kentucky Today
All the metrics in the weekly COVID-19 report from the Kentucky Department for Public Health increased as the omicron BA.5 subvariant continues to spread quickly across the state and the nation, and a new vaccine has been approved.
There were 15,884 new cases in the report issued July 25, an increase of 3,086 from last week, and up 6,310 from the June 27 report, four weeks ago.
There have been a total of 1,451,859 COVID-19 cases in Kentucky, since the first was reported on March 6, 2020.

In the one bright spot, the number of counties with 100 or more new cases in Monday’s report stood at 15. That is less than half of the 31 counties from last week. Jefferson had 1,512, Fayette 719, Hardin 305, Warren 280, Kenton 257, Boone 237, Madison 180, Daviess 164, Campbell 148, McCracken 143, Bullitt and Pike 134, Hopkins 111, Scott 106, and Boyd 103.
Public health officials report 59 more COVID-related deaths during the past week, compared to 49 a week ago, raising to 16,352 the number of Kentuckians lost since the start of the pandemic.
The positivity rate, which measures the percent of positive COVID tests jumped to 19.11% in the latest weekly report. That is up 1.41% from last week and is a 5.75% rise from four weeks ago.
The hospital census, like the rest of the COVID numbers this week, is also on the rise. There are currently 598 Kentuckians hospitalized, 11 more than last week and is 221 higher than four weeks ago. It’s a similar story in ICU and ventilator usage. Eighty-four patients are in intensive care and 33 on a ventilator, both of which are up slightly from last week. Compare that to four weeks ago when those numbers were 54 and 18, respectively.
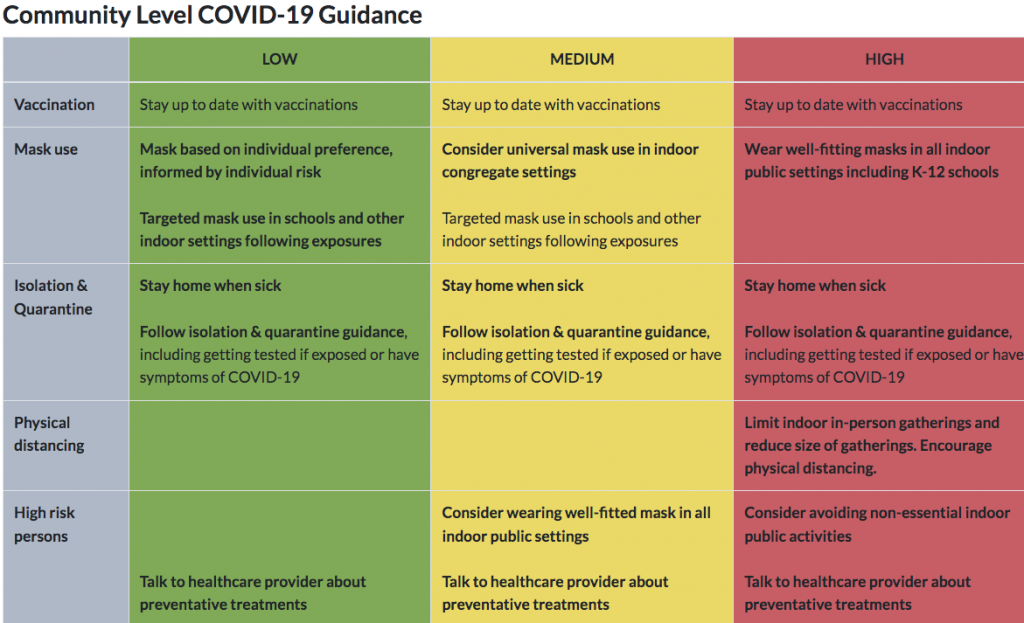
For more details and guidance, go to the state’s COVID-19 website, http://kycovid19.ky.gov/.
There is a new tool in the battle against COVID, as the U.S. Centers for Disease Control and Prevention announced Monday they have endorsed the Advisory Committee on Immunization Practices’ recommendation that the Novavax COVID-19 vaccine be used as another primary series option for adults ages 18 years and older, joining Pfizer and Moderna.
Unlike the other two, Novavax is a protein subunit vaccine, which packages harmless proteins of the COVID-19 virus alongside another ingredient called an adjuvant that helps the immune system respond to the virus in the future.
Vaccines using protein subunits have been used for over 30 years in the United States, beginning with the first licensed hepatitis B vaccine. Other protein subunit vaccines used in the United States today include those to protect against influenza and whooping cough.
Two doses of Novavax are given in the primary series, 3–8 weeks apart. Those who are moderately or severely immunocompromised should also receive 2 doses, given 3 weeks apart. There is no booster authorization yet.